Last Updated on May 8, 2025 by Ecologica Life
When you think of venom, you probably think of poison…You might think of venomous snakes and spiders. On first instinct, you probably don’t think of venom as a treatment for skin cancer. However, fascinating new research puts venom in a different light.
In this article, we look at venom-derived peptides and how exactly they have been used to treat melanoma.
Table of Contents
Introduction
In a groundbreaking study, researchers have discovered that peptides derived from the Brazilian tarantula and the Japanese horseshoe crab can effectively target and kill metastatic melanoma (skin cancer) cells, including those that are resistant to existing treatments.


Venom-Derived Peptides
The Brazilian tarantula and the Japanese horseshoe crab produce unique peptides in their venom. These peptides have been shown to selectively bind to and disrupt the membranes of melanoma cells without harming healthy cells.
This specificity offers a promising avenue for developing treatments that minimise the side effects associated with conventional therapies.
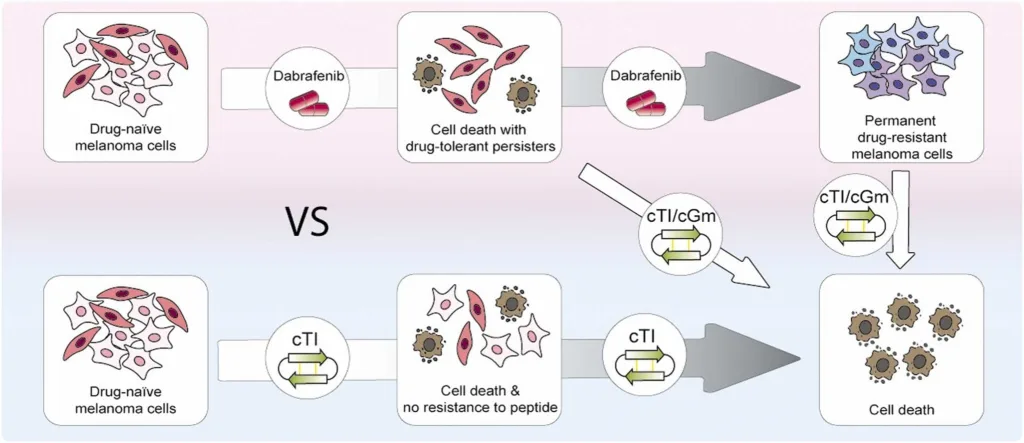
Benfield, A. H., Vernen, F., Young, R. S. E., Nadal-Bufí, F., Lamb, H., Hammerlindl, H., Craik, D. J., Schaider, H., Lawrence, N., Blanksby, S. J., & Troeira Henriques, S. (2024). Cyclic tachyplesin I kills proliferative, non-proliferative and drug-resistant melanoma cells without inducing resistance. Pharmacological Research, 207, 107298. https://doi.org/10.1016/j.phrs.2024.107298.
Licensed under CC BY 4.0: https://creativecommons.org/licenses/by/4.0/
In preclinical studies involving mouse models, these venom-derived peptides demonstrated significant efficacy in eliminating both active and dormant melanoma cells, including those that had developed resistance to standard treatments such as dabrafenib.
The ability to target dormant cells is particularly noteworthy, as these cells often evade traditional therapies and can play a role in cancer recurrence.
Why Does The Venom not Harm Normal Cells?
The reason that venom-derived peptides from the Brazilian tarantula and the Japanese horseshoe crab don’t harm normal cells lies in the differences in the structure and composition of cancer cell membranes compared to healthy cells.
Warning, Nerd Section Ahead
(Skip this part if you’re not interested in the nitty-gritty details).
Cancer cells, including metastatic melanoma, often have a higher negative charge on their outer membranes than healthy cells. This is due to the presence of certain molecules like phosphatidylserine, which are typically found on the inner membrane of healthy cells but are ‘flipped’ to the outer membrane in cancer cells.
This happens because cancer cells often exhibit disrupted membrane dynamics, the enzymes that keep phosphatidylserine on the inside – flippases – are downregulated or impaired.
The venom peptides are cationic, which means they are positively charged. This allows them to be selectively attracted to the negatively charged cancer cells, while largely ignoring the neutral membranes of healthy cells.
How Do Cancer Cells Develop Resistance and Why Don’t They Build Resistance to Venom Peptides?
Cancer cells are masters of adaptation. Most treatments – such as chemotherapy or targeted drugs – work by disrupting specific proteins or pathways inside the cell. Over time, cancer cells mutate, activate alternative pathways, or pump drugs out altogether. This makes them increasingly resistant to conventional therapies.
But venom peptides, like those from the Brazilian tarantula and the Japanese horseshoe crab, don’t play by the same rules. Instead of targeting proteins inside the cell, they attack the cell membrane directly by binding to negatively charged lipids that are abnormally exposed on cancer cells. This leads to rapid, physical destruction of the cell, rather than a slow biochemical shutdown.
Because this attack is broad, fast, and structural, it’s much harder for cancer cells to evolve around it. Rebuilding the membrane to avoid these peptides would come at a high cost – one that most cancer cells can’t afford. That’s why venom peptides are so promising: They go where other treatments fail, leaving cancer cells with nowhere to hide.
Implications for Cancer Treatment
This discovery opens up new possibilities for treating drug-resistant forms of melanoma, a skin cancer known for its aggressive nature and high mortality rate when it metastasises.
| Region | Incidence (per 100,000/year) |
|---|---|
| Australia/New Zealand | 31–42 |
| North America | 14–18 |
| Western Europe | 19 |
| UK | ~24 |
| US (all races) | 21.9 |
| US (Non-Hispanic White, M/F) | 39.7 / 26.8 |
| Africa/Asia | <1 |
By harnessing the natural components of venom, scientists hope to develop novel therapeutic strategies that could complement or enhance existing treatments.
Biodiversity in Medical Research
The use of venom-derived peptides highlights the importance of biodiversity in medical research. Some of our best medicines have all time have come from nature (e.g. penicillin, digitoxin). We should protect species, such as the Brazilian tarantula and the Japanese horseshoe crab, as nature is likely to hold the key to future medical breakthroughs.
This discovery highlights the intricate connection between environmental conservation and advances in human health and emphasises the need to preserve natural habitats and their inhabitants.